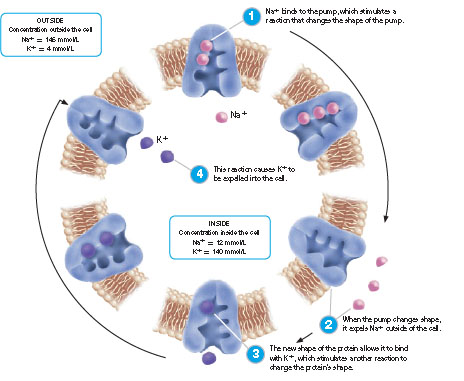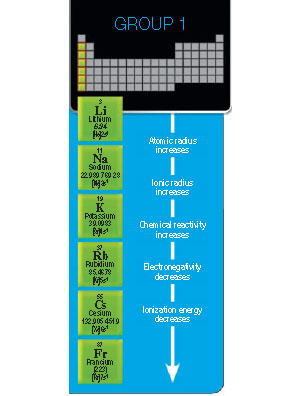
Characteristics
- do not occur in nature as free elements
- are reactive metals and are obtained by reducing the 1+ ions in their natural compounds
- are stored under kerosene or other hydrocarbon solvent because they react with water vapor or oxygen in air
- consist of atoms with one electron in the outermost energy level
- form colorless ions in solution, each of which has a 1+ charge
- form ionic compounds
- form water-soluble bases
- are strong reducing agents
- consist of atoms that have low ionization energies
- are good heat and electrical conductors
- are ductile, malleable, and soft enough to be cut with a knife
- have a silvery luster, low density, and low melting point

Lithium was discovered in 1817. It isfound in most igneous rocks and is used in batteries as an anode because it has a very low reduction potential. Lithium is soft and is stored in oil or kerosene to prevent it from reacting with the air.

Sodium derives its name from the word soda. It was first isolated in 1807 from the electrolysis of caustic soda, NaOH. Sodium is soft enough to be cut with a knife. It is shiny until it reacts with oxygen, which causes the surface to lose its luster.

Potassium was first isolated in 1807 from the electrolysis of caustic potash, KOH.